ABSTRACT
Introduction
Although an acute exercise session typically increases bone turnover markers (BTM), the impact of subsequent sessions and the interaction with preexercise calcium intake remain unclear despite the application to the “real-life” training of many competitive athletes.
Methods
Using a randomized crossover design, elite male rowers (n = 16) completed two trials, a week apart, consisting of two 90-min rowing ergometer sessions (EX1, EX2) separated by 150 min. Before each trial, participants consumed a high (CAL; ~1000 mg) or isocaloric low (CON; <10 mg) calcium meal. Biochemical markers including parathyroid hormone (PTH), serum ionized calcium (iCa) and BTMs (C-terminal telopeptide of type I collagen, osteocalcin) were monitored from baseline to 3 h after EX2.
Results
Although each session caused perturbances of serum iCa, CAL maintained calcium concentrations above those of CON for most time points, 4.5% and 2.4% higher after EX1 and EX2, respectively. The decrease in iCa in CON was associated with an elevation of blood PTH (P < 0.05) and C-terminal telopeptide of type I collagen (P < 0.0001) over this period of repeated training sessions and their recovery, particularly during and after EX2. Preexercise intake of calcium-rich foods lowered BTM over the course of a day with several training sessions.
Conclusions
Preexercise intake of a calcium-rich meal before training sessions undertaken within the same day had a cumulative and prolonged effect on the stabilization of blood iCa during exercise. In turn, this reduced the postexercise PTH response, potentially attenuating the increase in markers of bone resorption. Such practical strategies may be integrated into the athlete’s overall sports nutrition plan, with the potential to safeguard long-term bone health and reduce the risk of bone stress injuries.
Key Words: ENDURANCE, NUTRIENT TIMING, OSTEOCALCIN, PTH, Β-CTX-I, CALCIUM, BONE
Athletes often exhibit suboptimal bone health despite the well-known positive effects of exercise on bone (1). Immediate concerns include the effects of bone stress injuries on training consistency and availability for competition, whereas an increased risk of osteoporosis-related fractures may be seen later in an athlete’s career or after retirement (2,3). Such issues are known in a variety of sports such as cycling (4), dance (5), and distance running (6) and are seen among males and females (7). Key contributors toward bone health include energy availability, weight-bearing/bone loading exercise, and vitamin D (1,8). However, some athletes do consistently optimize these factors, and/or they may still require other measures to adequately support bone health. Therefore, there is interest in addressing other mechanisms by which bone metabolism may be perturbed. Elite rowers typically present with normal areal bone mineral density (aBMD) relative to general population norms; however, this may be inadequate to withstand the load required by the sport (9) and may be associated with history of bone stress injury of the rib (10), associated with significant training time loss and performance decrement (11).
A series of studies have identified that acute changes in serum ionized calcium (iCa) was associated with exercise correlate with bone turnover. For example, 2 h of moderate-intensity cycling in male cyclists was associated with a decrease in iCa and an elevation in parathyroid hormone (PTH) (12), suggesting that bone resorption may have been upregulated in an attempt to stabilize the reduction in iCa. It was hypothesized that increasing absorption of calcium from the gut during exercise, achieved by consuming calcium-rich foods or supplements before or during the session, might prevent bone resorption by providing an alternative calcium source. Indeed, preexercise calcium intake in elite male triathletes (13), as well as male (14) and female (15) cyclists, was found to suppress the cycling-associated rise in PTH and attenuated the increase in C-terminal telopeptide of type I collagen (β-CTX-1), a marker of bone breakdown, in some (13,15), but not all, protocols (14). The divergent outcomes may be due to differences in the source and timing of calcium intake (>60 vs 20 min prior). Studies in older individuals with lower levels of fitness who undertook brisk walking exercise also show similar responses to calcium support (16–18). These studies provide robust evidence that exercise disrupts calcium homeostasis, likely triggered by a decline in iCa and resulting in an increase in PTH and β-CTX-I.
The cause of the perturbation of iCa during exercise and whether this contributes to chronic and clinically meaningful changes in aBMD are still to be determined (19). The original hypothesis of dermal (sweat) mineral losses (20) was disproven in two different models. In young adults, there was no difference in exercise-associated iCa changes when brisk walking was undertaken in cool or warm conditions, despite differences in sweat/calcium losses. A more sophisticated methodology, involving the use of an intravenous calcium clamp to maintain iCa allowed more frequent blood sampling, including midexercise samples. Here, a drop in iCa was observed within the first 15 min of a 60-min cycling session at 80% heart rate (HR) maximum, well before significant sweat loss, with the calcium infusion attenuating the increase in PTH and CTX compared with the saline control (21). Phosphate has also been investigated given its involvement with PTH control at rest and exercise recovery; however, iCa seems more tightly linked with exercise-related PTH changes (22).
To date, studies of these perturbations in iCa and bone metabolism have focused on a single exercise session. However, high-performance athletes typically train multiple times a day, with subsequent sessions frequently commencing within 2–3 h, or before bone responses to the first session have normalized (15). The acute bone response to repeated exercise sessions has been briefly studied. Scott et al. (23) investigated the effect of the recovery period (3 vs 23 h) between two bouts of running on bone metabolism, before and during exercise on the trial days and for the following 4 d. Reducing the recovery time from 23 to 3 h did not alter the iCa, PTH, or β-CTX-I response to the second exercise bout or affect P1NP, a marker of bone formation that generally has a more chronic response to stimulus. In contrast, a very short recovery window (40 min) showed a blunting of the PTH response by the rest period (24). The response to preexercise calcium over multiple sessions has not yet been examined.
Together, these studies indicate a perturbation in bone turnover in response to exercise, likely triggered by a decline in iCa, resulting in a PTH-mediated increase in bone resorption. It occurs in a range of ages and fitness levels, between sexes, and in different modes and intensities of exercise. Preexercise calcium intake may maintain iCa and attenuate markers of bone resorption during and after the exercise session. Given the importance of deciphering the real-life significance of preexercise calcium supplementation, this study examined the effect of multiple sessions of exercise in a day (i.e., a typical training day for a high-performance athlete) bone turnover marker (BTM) in elite male rowers, using dietary opportunities to provide preexercise calcium supplementation.
METHODS
Participants
Eighteen elite male rowers from the Rowing Australia National Training Centre, Canberra, in preparation for potential Olympic representation, were recruited for this study and a parallel study investigating iron and hepcidin responses (25). One participant with newly identified food intolerances was excluded because of his inability to complete one of the dietary arms, whereas another was unable to complete the required training load because of recent illness. The final 16 participants are characterized in Table 1. Written informed consent was obtained from each athlete before study commencement. Ethics approval was obtained from the Australian Institute of Sport Ethics Committee (reference number: 20200905).
TABLE 1.
Subject characteristics.a
| Age: 26.3 ± 3.4 (20.7 to 32.4) yr | ||||
|---|---|---|---|---|
| Areal Bone Mineral Density | ||||
| aBMD (g·cm−2) | t Score | z Score | % Change from Previous Year | |
| Lumbar spine (L1–4) | 1.37 ± 0.10 (1.22 to 1.58) | 1.25 ± 0.82 (0.0 to 3.0) | 0.7 ± 0.8 (−0.4 to 2.5) | −0.3 ± 2.6 (−7.2 to 3.2) |
| Total hip | 1.15 ± 0.09 (0.98 to 1.35) | 0.47 ± 0.70 (−0.8 to 2.0) | −0.02 ± 0.75 (−1.4 to 1.6) | −0.4 ± 2.3 (−3.3 to 4.3) |
| Body Composition | ||||
| Height (cm) | Weight (kg) | TBLH Lean Mass (kg) | TBLH Body Fat (%) | TBLH Bone Mineral Content (kg) |
| 194.2 ± 3.7 (188.3 to 201.0) | 94.6 ± 4.6 (88.3 to 104.1) | 75.2 ± 4.0 (68.6 to 81.6) | 12.5 ± 2.9 (7.5 to 16.1) | 3.32 ± 0.28 (2.77 to 3.84) |
| Baseline Bloods | ||||
| Vitamin D (nmol·L−1) | TES (nmol·L−1) | fTES (pmol·L−1) | T3(pmol·L−1) | Cortisol (nmol·L−1) |
| 85.9 ± 17.3 (60.4 to 116.5) | 21.8 ± 4.0 (15.0 to 27.0) | 471 ± 97 (T) | 6.4 ± 0.5 (5.5 to 6.9) | 654 ± 103 (396 to 882) |
| Insulin (pmol·L−1) | Cortisol: Insulin | fTES: cortisol | IGF-1 (nmol·L−1) | LDL cholesterol (mmol·L−1) |
| 29.6 ± 13.3 (13.9 to 62.5) | 25.5 ± 10.0 (9.6 to 46.6) | 0.8 ± 0.3 (0.5 to 1.5) | 28.1 ± 6.6 (18 to 43) | 3.1 ± 1.1 (2.0 to 6.2) |
aValues are mean ± SD (range).
TBLH, total body less head.
Experimental overview
In a randomized, crossover design, athletes completed two trials, 1 wk apart, involving either a high (CAL) or low calcium (CON) dietary intervention (Fig. 1). Although it was not possible to make the intervention meals identical in appearance and taste, they were matched as closely as possible, and neither the athletes nor research personnel involved in data collection and entry were aware of allocation of the treatments. Subjects were tested on the same day of the week, with the weekly training prescription duplicated for both trials 1 and 2. On trial mornings, athletes arrived at the laboratory (0600–0700 h) in an overnight fasted, rested state, and a blood sample was collected from a cannula placed in a forearm vein (t = 0 min). Athletes then consumed either a low- (<10 mg) or high-calcium (1000 mg) standardized breakfast (t = 15 min). After 115 min, a preexercise whole blood sample was drawn, and 5 min later (t = 135 min), the first exercise session (EX1) commenced. Each exercise session comprised three 30-min sets on a rowing ergometer, separated by 5 min, and was designed to replicate a typical exercise session on water. Immediately after EX1, blood was sampled, and at t = 265 min (30 min after EX1 and 120 min before EX2), a second low- or high-calcium meal was consumed according to group allocation. Blood samples were drawn 1 and 2 h after EX1 (t = 295 and 355 min). At t = 385 min, a repetition of the earlier session was undertaken (EX2), noting a recovery period of 150 min between exercise bouts. Blood was collected at the break between the first and second sets of EX2 (t = 415 min, equating to 3 h after EX1). Blood was collected on completion of EX2 (t = 485 min), before the consumption of a recovery meal, with further samples at 1, 2, and 3 h after EX2 (t = 545, 605, and 665 min).
FIGURE 1.
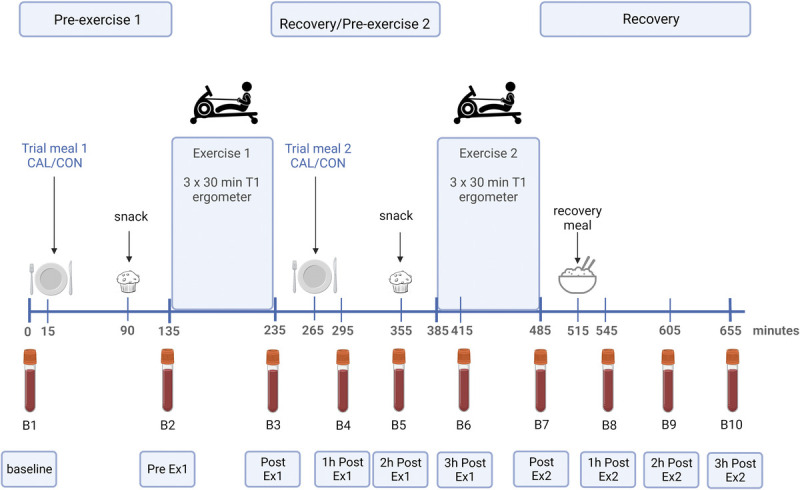
Trial overview including timing of feeding, exercise, and blood sampling. Created with BioRender.com.
Exercise sessions were completed on a rowing ergometer (Concept 2; Morrisville, VT), drag factor 130. Several rowers (n = 5), who had a current injury or injury risk undertook one of their 30-min exercise sets, in each of EX1 and EX2, on a Wattbike Pro cycling ergometer (Wattbike Ltd., Nottingham, United Kingdom), replicating real-world practice. Meanwhile, another participant undertook all trials on the Wattbike. Session intensity was individually prescribed at 90%–100% of the power previously identified from an incremental test as the point at which capillary blood lactate reached 2 mmol·L−1. Mean power was recorded for each effort, as was HR (in beats per minute; Wahoo Tickr X; Wahoo Fitness, Atlanta, GA) and subjective rating of perceived exertion (RPE) according to the modified Borg scale (6–21,26).
Bone mineral density and body composition
Body composition and aBMD (anterior posterior (AP) spine (L1–L4), proximal femur) were measured by dual x-ray absorptiometry in the morning, fasted and rested, according to methods described previously (27) (GE Healthcare, Lunar iDXA, Encore v16.2). Because the subjects were too tall for the scanning bed, body composition was assessed excluding the head as previously described (28).
Dietary standardization
Subjects followed a standardized diet for the 24 h before each of the two experimental trial days, individualized by a sports dietitian according to body mass, habitual diet, and intolerances and following recommendations by Jeacocke and Burke (29). This diet was provided to subjects as prepackaged food items with verbal and written instruction to direct intake. The nutrient prescription for these diets was 256 kJ·kg−1 body mass, energy, 8 g·kg−1 carbohydrates (CHO), 2–3 g·kg−1 protein, and 35% of energy from fat. Substitutions were made as required for gluten-free (n = 1), lactose-intolerant (n = 1), and other food sensitivities (n = 1) while retaining the macronutrient targets. Abstinence from alcohol intake and habitual/ad libitum intake of caffeine and fluid intake were maintained, with recording of intake to allow replication for each trial. Subjects were permitted to consume additional foods according to hunger on the first week, with a checklist being provided to note any deviations, which allowed replication during the second trial. These were checked on arrival for both trial mornings by the dietitian.
On the trial day, food was prepared, served, and consumed from the onsite kitchen according to the trial schedule. Subjects consumed the same meals and snacks for each trial day except for the targeted preexercise intervention meals. The interventions consisted of a CAL (high calcium, 1000 mg) or CON (low calcium, <10 mg calcium) menu of Bircher muesli and a toasted sandwich. For CAL, dairy foods were the primary contributors to the calcium target and easily met this 1000-mg goal, within portion sizes typically consumed in a breakfast meal in this population (Table 2). For CON, a nondairy yoghurt, nonfortified almond milk and vegan cheese were used as substitutes. In designing these menus, priority was given first to achieving calcium targets, then to matching CHO and energy content of the meals with the protein and fat matched as closely as possible. At 75 min after a meal, all participants consumed a preexercise snack (muffin). During the exercise sessions, water was provided ad libitum and recorded accordingly. Body mass was measured before and after exercise to allow estimation of fluid losses through sweat (with adjustment for the volume of fluid consumed and any urine losses during the session). A CHO-rich gel (Science in Sport PLC, London, United Kingdom) or CHO-equivalent quantity of confectionary was consumed (~30 g CHO) during each 5-min break between exercise sets. To maintain real-life practice, without disturbing the study intervention, athletes were able to request more (“calcium-free” <10 mg) food in the recovery period between exercise sessions in addition to the preexercise meal. Any additional foods consumed in the first trial were recorded and repeated in the second trial. The nutrient composition of all diets was calculated using a computerized dietary analysis package (Nutritics Ltd., Dublin, Ireland) by the same sports dietitian.
TABLE 2.
Trial day diet intake: low (CON) and high calcium (CAL) trials from waking to the conclusion of the trial.
| Trial | |||
|---|---|---|---|
| CON | CAL | P | |
| Energy (kJ) | 19,295 ± 2100 | 20,345 ± 1600 | <0.001* |
| Energy (kJ·kg−1) | 200 ± 23 | 211 ± 22 | 0.001* |
| Protein (g) | 193 ± 31 | 220 ± 22 | 0.002* |
| Protein (g·kg−1) | 2.0 ± 0.3 | 2.3 ± 0.3 | 0.003* |
| Fat (g) | 151 ± 16 | 163 ± 14 | 0.003* |
| Fat (g·kg−1) | 1.6 ± 0.2 | 1.7 ± 0.2 | 0.004* |
| CHO (g) | 614 ± 69 | 615 ± 69 | 0.89 |
| CHO (g·kg−1) | 6.4 ± 0.8 | 6.4 ± 0.8 | 0.98 |
| Calcium (mg) | 224 ± 160 | 2319 ± 160 | <0.001* |
Values are presented as mean ± SD.
*Significant difference between trials (P < 0.05).
Thirty minutes after the completion of the first exercise session, the preexercise meal and snack were provided in the same sequence before the second exercise session. On completion of this session, all participants consumed a recovery meal (butter chicken or beef and black bean, rice, and vegetables) and snack (chocolate chip cookie). The quantity of this meal was self-selected in trial 1, recorded and replicated in trial 2.
Blood analysis
During each trial, 10 venous blood samples were collected into either 6- or 8-mL serum separator tubes (BD Vacutainer, Macquarie Park, Australia). Blood samples were taken at rest (fasted), before exercise (2 h after breakfast), and immediately, 1 h, 2 h, and 3 h after each exercise session (Fig. 1). Samples were left to clot for 30 min before being centrifuged at 1500g for 10 min at 4°C. Serum was aliquoted into 1.5-mL cryotubes and frozen at −80°C until batch analysis was performed. PTH and vitamin D (25-hydroxyvitamin D, or 25(OH)D) concentrations were measured by chemiluminescent immunoassay (Access 2; Beckman Coulter, Brea, CA), with coefficients of variance of (CV) 4.5% and 6.5%, respectively. CTX concentrations were assessed by electrochemiluminescence immunoassay (Cobas e411; Roche Diagnostics, Basel, Switzerland), with a CV of 4.2%. Total osteocalcin (tOC) and undercarboxylated osteocalcin (ucOC) concentrations were measured by an automated, noncompetitive, chemiluminescent immunoassay performed on a Cobas e801 (Roche Diagnostics), with CV values of 1.2% and 3.2%, respectively. Metabolic and reproductive hormones, free triiodothyronine (T3), total (TES) and free testosterone (fTES), cortisol, and insulin-like growth factor 1 (IGF-1) were assessed by a commercial laboratory (Laverty Pathology, Bruce, ACT, Australia). IGF-1 was assayed using the DiaSorin Liason® XL (DiaSorin Diagnostics, Sallugia, Italy).
Capillary whole blood was used to determine ionized calcium, hemoglobin, and hematocrit (Hct) with the point-of-care i-STAT device (Abbott Point of Care Inc., Princeton, NJ), with a CV of 1.5% and a sensitivity of 10%. Changes in BTM were adjusted for hemoconcentration using methods described by Van Beaumont et al. (30). Raw Hct readings were multiplied by a factor (0.96 × 0.91) to correct for plasma trapped between red blood cells and to convert venous Hct to whole-body Hct, respectively. BTM concentrations were adjusted to the concentration expected (CE) based on fluid shifts alone (equation 1), where changes in Hct from immediately preexercise (Hct1) to all postexercise values (Hct2) were calculated (with C1 representing the initial concentration of the biomarker).
| [1] |
Second, the unadjusted postexercise biomarker concentrations (C2) were corrected for CE (equation 2), giving an Hct-corrected concentration (C2Hct).
| [2] |
All values are reported as both adjusted for hemoconcentration (indicated by adj) and unadjusted (indicated by unadj).
Statistical analysis
Statistical analysis was performed using R Studio (R Core Team, 2021, v3.5.2). Linear mixed models were constructed to assess changes in Hct, adjusted and unadjusted iCa, PTH, β-CTX-I, and tOC, ucOC, and ucOC:tOC, with fixed effects for time and condition, and subject identification and week, used as random effects. Similar models were used to determined changes in training variables (power output, HR, and RPE). Sweat loss was included as a covariate in the analysis of β-CTX-Iadj to rule out any association with dermal calcium losses. Visual inspections of residual plots were used to assess homoscedasticity and normality. Significant deviations were noted for PTHadj and β-CTX-Iadj, and thus, data were log-transformed for analysis. Statistical significance of the fixed effects was determined using type II Wald tests with Kenward–Roger degrees of freedom. Where significant fixed effects were evident, Tukey’s post hoc comparisons were performed to identify specific condition differences. Finally, the relationship between aBMD (AP spine, proximal femur, and z scores) and β-CTX-Iadj (preexercise concentrations, percentage change at 1 h postexercise from preexercise for EX1 and EX2) was assessed via Pearson’s correlations. Pretrial and trial day dietary intake were compared with paired-sample t tests. Significance was set at P < 0.05.
RESULTS
Subject characteristics
Characteristics of the elite male rowers who participated in this study are summarized in Table 1. The group consisted of tier 5/world-class (n = 10), tier 4/international (n = 4), and tier 3/national (n = 2) level athletes as defined by McKay et al. (31). aBMD was normal in most participants, with two subjects classified as low bone density for age in an athlete (z < −1) for the proximal femur and none for the AP spine. Six of the athletes had insufficient 25(OH)D levels (<80 nmol·L−1), but none were deficient (<50 nmol·L−1) (32). Hormonal biomarkers of low energy availability (e.g., IGF-1, TES, and T3) were within reference ranges, with the exception of cortisol for which 11 participants had a value above the reference range for morning blood samples (>620 mmol·L−1). However, the fTES to cortisol ratio was not below the threshold indicative of overstrain for the group (33).
Dietary intake
Dietary intake during the 24-h pretrial standardization period was well matched, with energy intakes of 21,985 ± 5500 versus 21,660 ± 7300 kJ (P = 0.82), CHO intakes of 6.8 ± 1.5 versus 7.2 ± 1.7 g·kg−1 body mass (P = 0.048), protein intake of 2.6 ± 0.6 versus 2.7 ± 0.6 g (P = 0.08), and fat intake of 182 ± 58 versus 195 ± 54 g (P = 0.08). Trial day analysis (all food and fluid consumed until the cessation of the trial) revealed no differences in intakes of CHO, but slightly greater intakes of energy, protein, and fat in the calcium trial (Table 2). Although statistically significant, these differences related to the small differences in the foods chosen in the final postexercise recovery meal (time point, 515 min; Fig. 1) and not the preexercise CON and CAL meal. As such, these differences are unlikely to create clinically significant effects on trial outcomes (~10 kJ·kg−1, 0.3 g·kg−1 protein, 0.1 g·kg−1 fat). CON and CAL intervention meals are described in Table 3.
TABLE 3.
Intervention meal.
| Intervention | Meal Components | Nutrient Breakdown |
|---|---|---|
| CON (low calcium meal) | Low-calcium Bircher muesli: Nutty Bruce Roasted Almond & Oat Milk (200 mL) Kingland Dairy Free Greek Style Yoghurt Mixed Berry (170 g) Carman’s Bircher Muesli (90 g) Low-calcium toasted sandwich: Helga’s Mixed Grain Bread (2 slices) Ham (100 g) Butter (9.5 g) My Life Bio Cheese (40 g) |
Energy: 4819 kJ Protein: 49 g Fat: 50 g CHO: 127 g Calcium: 1 mg |
| CAL (high calcium meal) | High-calcium Bircher muesli: Pauls High Calcium Milk (200 mL) Siggis High Calcium Vanilla Yoghurt (125 g) Carmans Bircher Muesli (90 g) High-calcium toasted sandwich: Helga’s Mixed Grain Bread (2 slices) Ham (50 g) Butter (9.5 g) Bega Tasty Cheese (50 g) |
Energy: 4819 kJ Protein: 61 g Fat: 52 g CHO: 108 g Calcium: 1042 mg |
Exercise sessions
Mean power output was 260 ± 5 W for CON and 255 ± 5 for CAL, representing 101% ± 2% and 99% ± 2% of prescribed T1 power, respectively, with no significant differences between interventions (P = 0.27) or sessions (EX1 vs EX2, P = 0.35). No significant differences between trials or sessions for power or HR were recorded. Although there were no differences in RPE between trials, EX2 was perceived as marginally more strenuous than EX1 (CON 11 ± 0.4 vs 12 ± 0.4, CAL 11 ± 0.4 vs 12 ± 0.4, P = 0.002). Mean sweat loss was 1306 ± 332 mL·h−1 of exercise and was not different between CON and CAL (P = 0.35) or related to aBMD or β-CTX-I.
Markers of bone remodeling
There was a main effect of treatment on Hct (Fig. 2A), with values being ~1.8% higher in CON than CAL condition (P < 0.001). Although significantly different, the difference was unlikely to be practically important given the magnitude of changes seen with exercise. There was a main effect of time (P < 0.001), with Hct increasing from baseline over the course of EX1, being ~2.3% higher at the finish of this session and then falling during recovery by 7.8%, before repeating this pattern for EX2.
FIGURE 2.
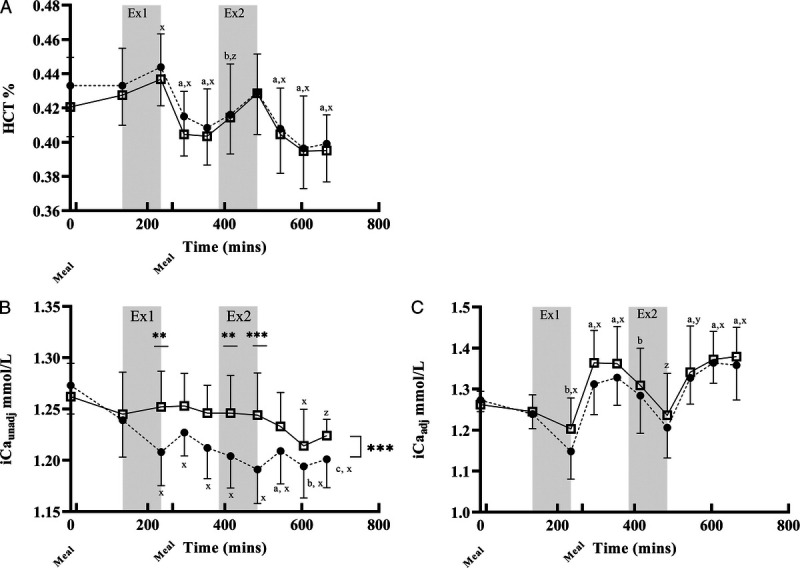
HCT and iCa for calcium and control treatments. Main effects for time, treatment, or interactions. *P < 0.05, **P < 0.01, P < 0.001; x, y, and z indicate P < 0.001, P < 0.01, and P < 0.05, respectively, relative to B1. A, B, and C indicate P < 0.001, P < 0.01, and P < 0.05, respectively, relative to B2. Data are presented mean ± SD. Graph C is adjusted for hemoconcentration (adj). A, HCT: CON > CAL (P < 0.001), time effect (P < 0.001), no treatment–time interaction. B, iCaunadj: CAL > CON (P < 0.001). Treatment–time interaction (P < 0.001), CAL > CON for B3, B6, and B7. C, iCaadj: CAL > CON (P < 0.01), effect of time (P < 0.0001). There was no time–treatment interaction.
iCaunadj concentrations (Fig. 2B) were higher for CAL than CON (P < 0.001), with a main effect of time (P < 0.001). The treatment–time interaction (P < 0.001) demonstrated a gradual drop in iCaunadj over the course of EX1 and EX2 in CON (P < 0.001), whereas concentrations were maintained in the CAL trial until 2 h after EX2 (P = 0.0003). Compared with the CAL trial, iCaunadj concentrations with CON were ~4.5% and 2.4% lower at the end of EX1 and EX2, respectively. iCaunadj concentrations were significantly higher in CAL than CON for B3 (post-EX1, P = 0.002), B6 (3 h post-EX1, P = 0.005), and B7 (post-EX2, P < 0.0001; Fig. 2B). There was a main effect of treatment for iCaadj concentrations (Fig. 2C), with values being higher in CAL than CON (P < 0.005). Furthermore, there was a decrease in iCaadj over each exercise session and an increase during recovery in both conditions (P < 0.001).
Adjustment for hemoconcentration did not alter the interpretation of BTM (Figs. 3, 4), and as such, discussion of findings is limited to those for adjusted markers. Mean concentrations of PTHadj (Fig. 3B) were higher in CON than CAL (P < 0.001). Although PTHadj concentrations did not change from baseline in the CAL trial, the time–treatment interaction (P < 0.05) showed increased concentrations of PTHadj in the CON trial for all time points after the start of EX1, except for 1 h post. There was a main effect of time (P < 0.001) for β-CTX-Iadj concentrations, with the first meal being associated with a decrease from fasting concentrations. There was a treatment effect, with β-CTX-Iadj concentrations being higher with CON than CAL (P < 0.001). The time–treatment interaction demonstrated a maintenance of β-CTX-Iadj in the CAL trial until the first hour of recovery after EX2. Meanwhile, in CON, there was a pronounced increase over each exercise session, with β-CTX-Iadj concentrations being higher than in the CAL trial from the completion of EX1 (B3) until 2 h after EX2 (B9).
FIGURE 3.
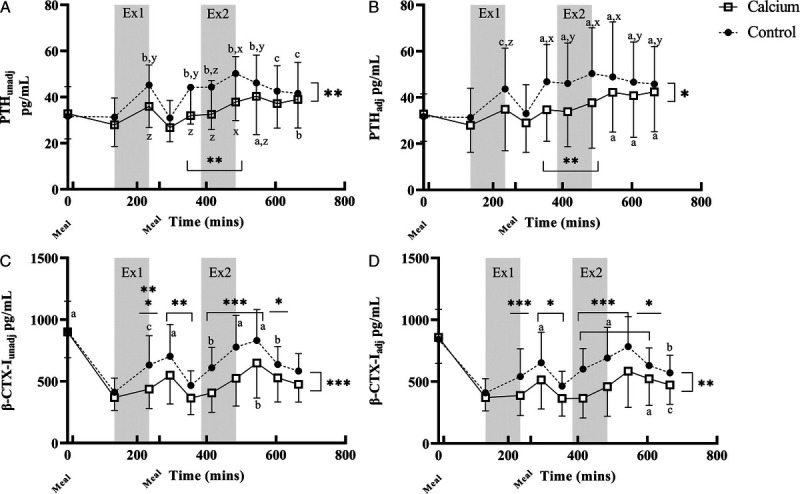
PTH and β-CTX-I for calcium and control. Main effects for time, treatment, or interactions. *P < 0.05, **P < 0.01, P < 0.001; x, y, and z indicate P < 0.001, P < 0.01, and P < 0.05, respectively, relative to B1. A, B, and C indicate P < 0.001, P < 0.01, and P < 0.05, respectively, relative to B2. Data are presented mean ± SD. Graphs B and C are adjusted (adj) for hemoconcentration. Statistical analysis was performed on log-transformed data. A, PTHunadj: CON > CAL (P < 0.001), effect of time (P < 0.001), treatment–time (P < 0.01), CON higher than CAL for B5–B7 (P < 0.001). B, PTHadj: CON > CAL (P < 0.0001), effect of time (P < 0.0001), treatment–time (P < 0.05), CON higher than CAL for B5–B7 (P < 0.001). C, β-CTX-Iunadj: CON > CAL (P < 0.0001), effect of time (P < 0.0001), treatment–time (P < 0.0001), CON > CAL for B3 and B6–B8 (P < 0.001), B4–B5 (P < 0.01), and B9 (P < 0.050. CAL B1 higher than all other time points (P < 0.0001); CON B1 higher than all other time points except B7 and B8 (P < 0.001). D, β-CTX-Iadj: CON > CAL (P < 0.001), effect of time (P < 0.0001), treatment–time (P < 0.0001), CON > CAL for B3 and B6–B8 (P < 0.001), B4–B5 and B9 (P < 0.05). CAL B1 higher than all other time points (P < 0.0001); CON B1 higher than all other time points except B7–B9 (P < 0.05).
FIGURE 4.
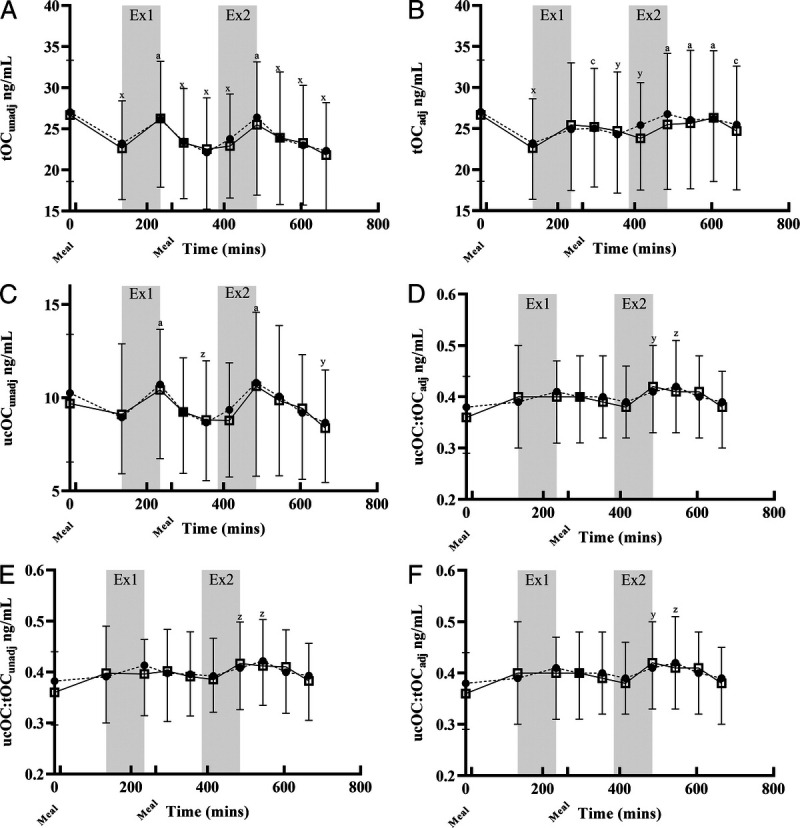
Osteocalcin: no effect of treatment was seen on any measure of osteocalcin. *P < 0.05, **P < 0.01, P < 0.001; x, y, and z indicate P < 0.001, P < 0.01, and P < 0.05, respectively, relative to B1. A, B, and C indicate P < 0.001, P < 0.01, and P < 0.05, respectively, relative to B2. Data are presented mean ± SD. Graphs B, D, and F are adjusted for hemoconcentration (adj). A, tOCunadj: effect of time (P < 0.001). B, tOCadj: effect of time (P < 0.001). C, ucOCunadj: effect of time (P < 0.001). D, ucOCadj: effect of time (P < 0.001). E, ucOC/tOCunadj: effect of time (P < 0.01). F, ucOC/tOCadj: effect of time (P < 0.01). Calcium and Control.
Concentrations adjusted for Hct changes of tOC (tOCadj; Fig. 4B), ucOC (ucOCadj; Fig. 3B), and ucOCadj/tOCadj (Fig. 4D) were unaffected by preexercise calcium intake. tOCadj decreased after feeding (P < 0.0001), increased during EX2 (P < 0.001), and decreased in the recovery period from both sessions (P < 0.05 and P < 0.001 for EX1 and EX2, respectively). ucOCadj was highest during the recovery period from EX2 (P < 0.001). Similarly, ucadj/tOCadj was slightly increased from pre-EX1 to post-EX2 (P < 0.01 and P < 0.05 for immediately and 1 h post, respectively; Fig. 4F).
DISCUSSION
This is the first study to investigate the effect of repeated, strenuous, non–weight-bearing exercise sessions, undertaken in close succession, on BTM in elite athletes. A further novel feature involved the intake of a calcium-rich meal before each session to potentially offset the exercise-associated perturbations to calcium homeostasis. The main findings were the following: (i) the control trial, involving minimal (<10 mg calcium in the preexercise meal) was associated with a drop in iCa concentrations with each exercise session. Meanwhile, the intake of a calcium-rich meal (~1000 mg calcium) before each session enabled a near maintenance of serum iCa concentrations, with iCa values being higher in the CAL trial than the CON trial for a period of ~7 h spanning the two training sessions and postexercise recovery. (ii) The perturbation of iCa in the CON trial was associated with an elevation of serum PTH (PTHadj) and the marker of bone resorption, β-CTX-Iadj, during exercise and recovery, with an apparent accentuation of these changes after the second exercise session. (iii) The effect of a calcium-rich preexercise meal in countering exercise-associated reductions in blood calcium concentrations in a single exercise session seems to be repeatable and reduced PTHadj and β-CTX-Iadj concentrations over a sustained (7-h) time period. We conclude that athletes who undertake repeated sessions of non–weight-bearing activity in close succession daily may be exposed to prolonged periods favoring bone resorption. However, our dietary intervention, shown to be practical to achieve and commensurate with other nutritional goals of elite male athletes, may support bone health by stabilizing the conditions that would otherwise favor bone turnover for a significant portion of the day. Although this effect has previously been described in response to a single exercise session (13,14,34), we now show that it has particular relevance to the “real-life” training of many competitive athletes.
Rowers have been identified as a high-risk group for bone-related injuries, with rib stress injuries being the highest burden injury, occurring in 16% of an elite rowing population over two Olympiads and resulting in 19.6 of the lost athlete training days (35). Harris et al. (10) identified that athletes with a rib stress injury during the Rio Olympic cycle (2013–2016) failed to win an international medal in the year of their injury or at the Olympic Games. Compromised aBMD for spine (36–38) and total body measures (36) has been reported in rowers, particularly lightweight classes who are most at risk of low energy availability due to weight-making strategies. Although there is the potential for aBMD to be above populations standards, it may not be sufficient to withstand the repetitive nature of high-volume training (38). Factors associated with bone stress injury in this population have previously been identified as low aBMD, low energy availability, and insufficient daily calcium intake, with vitamin D and K status found to be relatively less important (Lundy et al., unpublished data). In this current study cohort, risk of low energy availability was considered low, based on the associated biomarkers measured. Indeed, we found a marginally elevated cortisol as the only abnormality, which was likely explained by the implementation of the study immediately after a short break in the training season. However, if ongoing, elevated cortisol may contribute toward bone loss (39) independent of low energy availability. Given the importance of rib stress injury to performance, all aspects of bone health support strategies need to be considered.
Bone is a dynamic tissue that is constantly underdoing resorption and formation with the balance between activities contributing toward overall bone health (40). However, previous studies of athletes (4,41) have suggested that some exercise activities may create a perturbation to bone turnover favoring increased resorption causing loss of aBMD over time. Specifically, non–weight-bearing exercise, such as rowing, where there is reduced stimulus of bone formation from mechanical loading at some sites may be of concern. The current study adds to the robust evidence of a reduction in blood concentrations of ionized or free calcium, via an unknown mechanism at the commencement of endurance exercise. This triggers a homeostatic response to stabilize blood calcium via an acute PTH-mediated resorption of bone (14,21,24). Here, CTX-I, released from osteoclasts during the breakdown of collagen fibrils and appearing in the blood stream as β-CTX-I, can be considered an acute marker of bone resorption (40). Previous studies have proposed that this process may expose some athletes to repeated and lengthy periods in which there is elevation of bone resorption (i.e., the duration of their training sessions and the ~2 h of reequilibration of markers of bone turnover) (15). Our interest in elite rowers, and indeed, the design of this study, draws attention to the high-volume training programs of many endurance athletes in which two or three strenuous exercise sessions are undertaken each day, with subsequent sessions often commencing within the window before apparent restoration of equilibrium of bone turnover has occurred. Although our study is unable to determine the cumulative impact of these changes over time, it confirmed that each session was associated with a perturbation to blood iCa with downstream effects on PTHadj and β-CTX-Iadj that suggested an extended (~7 h) period of elevated bone resorption. Although we acknowledge that our study relies on relatively acute changes to BTM, we suggest that these results warrant further investigation of the timing and nature of exercise sessions on bone turnover. Furthermore, we propose that strategies to attenuate the initial perturbation of blood iCa may help to support bone health in these scenarios.
The provision of an alternative source of calcium to buffer exercise-mediated blood calcium losses has been achieved via intravenous clamps in research settings (18,21) as well as the gut release of calcium ingested from supplements (14,34) or foods (15) in more real-world protocols. Here, the timing of intake of calcium seems to be important with studies that have used oral calcium either in close proximity or during exercise showing unclear effects of supplementation, particularly on β-CTX-I (14,34). The optimal dose of preexercise calcium has not been identified, and future investigation should target this issue. Nevertheless, previous work from our group found that the intake of a calcium-rich (1200 mg) meal, consumed 2 h before a cycling session, represented a protocol that integrated gastrointestinal comfort (42), preexercise fuel goals, and an effective dietary source of calcium to address the exercise-mediated changes to iCa (15). The current study confirmed that everyday foods can provide a practical intake of 1000 mg of calcium while simultaneously achieving energy, macronutrient and micronutrient goals for this athletic population. The menu, based on dairy foods, was easily consumed and could be adapted for an individual with lactose intolerance, although not suited to vegan eaters. The timing and size of the meal contributed to the fuel goals for each session as well as daily energy requirements, and our anecdotal observations of good gastrointestinal tolerance are supported by previous systematic measurements of gastrointestinal comfort when a similar meal was consumed before a sustained high-intensity exercise time trial (15). Importantly, the meal was able to stabilize blood iCa concentrations during and after exercise. Indeed, iCa remained elevated above that of the CON diet for ~7 h, spanning the period from the start of the first exercise session until 2 h of recovery after EX2. The preexercise calcium-rich meal was equally effective in preventing the decline in iCa over exercise in each session, showing that the effect can be repeated.
The improved maintenance of iCa with the CAL trial was associated with lower PTHadj concentrations over the trial day, and an attenuation of the increase in PTHadj associated with exercise, particularly around the second session. The duration and periodicity of exposure to elevations in PTH govern the net effect on bone mass, with an intermittent increase in PTH stimulating bone formation, whereas prolonged continuous exposure to high levels tips the balance in favor of resorption (43). In clinical situations, PTH concentrations should be assessed in conjunction with 25(OH)D status and calcium intake, as vitamin D deficiency has a secondary effect on PTH (44). We note that 25(OH)D levels were insufficient in 30% of our sample, likely because of the timing of our study coinciding with the annual seasonal nadir. Nevertheless, this is unlikely to have affected our findings given the crossover design of the study and the absence of frank deficiency within our group.
Although further investigation is needed, it is likely that the PTHadj response to the CAL supplementation in the current study is indicative of less bone resorption (22). Indeed, the increase in β-CTX-Iadj seen with CON was cumulative and relatively prolonged, spanning the first exercise bout until 2 h after the final exercise session was completed, a period of 6–7 h. In contrast, β-CTX-Iadj was unchanged from baseline for CAL and was higher at only two time points, a period spanning around 2 h. Our findings of an attenuation of the β-CTX-Iadj response to exercise after calcium supplementation is in agreement with the results of several studies of single exercise bouts in which the timing of calcium intake has been designed to allow gut release during the early exercise period (13,15,18,21).
Our study included blood measurements of osteocalcin, a protein thought to be primarily synthesized by osteoblasts and often used as a marker for bone turnover (45). Indeed, we investigated both tOC concentrations (tOCadj), which includes the vitamin K–stimulated carboxylated form (cOCadj) with high bone affinity, and its undercarboxylated form (ucOCadj), which is receiving attention for a range of endocrine effects (46). In the current study, we observed small but significant increases in tOCadj and ucOCadj associated with each exercise bout and a minor increase in the ratio of ucOCadj/tOCadj after the second bout, but no differential effect of the preexercise calcium intake on these changes. The acute postexercise response is consistent with previous research (47,48), with the lack of difference between CAL and CON supporting the observation that OC is likely most influenced by perturbations to energy and CHO availability during exercise (49,50), which were matched in the present study, rather than acute calcium concentrations.
We acknowledge the limitations of this study, including the focus on elite male athletes (necessitated by COVID-19 restrictions on the interstate travel of their female counterparts), as well as the small but clinically insignificant differences in the macronutrient and energy intake of the trial day diets. We also note the reliance on systemic markers of bone turnover, which may provide an acute picture of change but not the long-range implications. We also note that procollagen type I N-terminal propeptide is considered the preferred marker of bone formation (40), but the time course of interesting change for this marker (days vs hours) (51) was incompatible with the current protocol. Indeed, in the previous study from our group involving highly trained cyclists (15), preexercise Ca intake had no effect on the PINP response to exercise, despite a clear attenuation of the increase in CTX-1. Nevertheless, we identify many strengths including the involvement of elite athletes, the real-world application of the study theme to many highly trained athletes, and the involvement of holistic dietary strategies to address impairments of bone remodeling that are of relevance to health and performance. Future research could monitor changes to bone formation markers over the days after exercise and better determine if an attenuation of β-CTX-I is likely to have a positive impact on long-term bone outcomes.
CONCLUSIONS
In summary, the findings of this study suggest that preexercise intake of calcium-rich foods can lower markers of bone resorption during and after exercise. Specifically, the repeated intake of calcium-rich meals before training sessions undertaken within the same day has a cumulative and sustained effect on the stabilization of blood iCa during exercise. In turn, this reduces the PTH response to exercise, likely leading to attenuating the increase in markers of bone resorption. Such practical strategies may be integrated into the athlete’s overall sports nutrition plan, with the potential to safeguard long-term bone health and reduce the risk of bone stress injuries.
Acknowledgments
We thank the athletes and coaches involved in this study for their commitment and assistance. Specific thanks to Dr. Tony Rice, Dr. Sally Clark, Dr. Kristin Everett, Dr. Nathan Versey, Samantha Lewis, and Kate Gemmell for their assistance with data collection.
This study was funded by the Mary McKillop Institute for Health Research, Rowing Australia, and Wu Tsai Human Performance Alliance. The salary of Marc Sim is supported by a Royal Perth Hospital Research Foundation Career Advancement Fellowship (CAF 130/2020) and an Emerging Leader Fellowship from the Western Australian Future Health Research and Innovation Fund. The authors declare that they have no conflicts of interest. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Authorship: The study was designed by L. M. B., A. K. A., B. L., and N. F. Data were collected by L. M. B., A. K. A., N. T., B. L., B. A., A. M., and M. R., and analyzed by B. L., L. M. B., A. K. A., N. T., and N. F.; data interpretation and manuscript preparation were undertaken by L. M. B., A. K. A., and B. L. All authors contributed to the writing and approved the final version of the manuscript.
Contributor Information
BRONWEN LUNDY, Email: blundy@rowingaustralia.com.au.
ALANNAH K. A. MCKAY, Email: alannah.mckay@acu.edu.au.
NIKITA C. FENSHAM, Email: nikita.fensham@myacu.edu.au.
NICOLIN TEE, Email: nicolin.tee@acu.edu.au.
BRYCE ANDERSON, Email: bryceanderson@live.com.au.
AIMEE MORABITO, Email: aimee.morabito@ausport.gov.au.
MEGAN L. R. ROSS, Email: meganlrross11@gmail.com.
MARC SIM, Email: marc.sim@ecu.edu.au.
KATHRYN E. ACKERMAN, Email: kathryn.ackerman@childrens.harvard.edu.
REFERENCES
- 1.Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR. American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc. 2004;36(11):1985–96. [DOI] [PubMed] [Google Scholar]
- 2.Mackinnon AL Jackson K Kuznik K, et al. Increased risk of musculoskeletal disorders and mental health problems in retired professional jockeys: a cross-sectional study. Int J Sports Med. 2019;40(11):732–8. [DOI] [PubMed] [Google Scholar]
- 3.Myburgh KH, Hutchins J, Fataar AB, Hough SF, Noakes TD. Low bone density is an etiologic factor for stress fractures in athletes. Ann Intern Med. 1990;113(10):754–9. [DOI] [PubMed] [Google Scholar]
- 4.Barry DW, Kohrt WM. BMD decreases over the course of a year in competitive male cyclists. J Bone Miner Res. 2008;23(4):484–91. [DOI] [PubMed] [Google Scholar]
- 5.Amorim T Koutedakis Y Nevill A, et al. Bone mineral density in vocational and professional ballet dancers. Osteoporos Int. 2017;28(10):2903–12. [DOI] [PubMed] [Google Scholar]
- 6.Pollock N Grogan C Perry M, et al. Bone-mineral density and other features of the female athlete triad in elite endurance runners: a longitudinal and cross-sectional observational study. Int J Sport Nutr Exerc Metab. 2010;20(5):418–26. [DOI] [PubMed] [Google Scholar]
- 7.Changstrom BG, Brou L, Khodaee M, Braund C, Comstock RD. Epidemiology of stress fracture injuries among US high school athletes, 2005–2006 through 2012–2013. Am J Sports Med. 2015;43(1):26–33. [DOI] [PubMed] [Google Scholar]
- 8.Sale C, Elliott-Sale KJ. Nutrition and athlete bone health. Sports Med. 2019;49(2 Suppl):139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonvik KL, Torstveit MK, Sundgot-Borgen JK, Mathisen TF. Do we need to change the guideline values for determining low bone mineral density in athletes? J Appl Physiol (1985). 2022;132(5):1325–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lundy B, Suni V, Drew M, Trease L, Burke LM. Nutrition factors associated with rib stress injury history in elite rowers. J Sci Med Sport. 2022;S1440-2440(22):246–8. [DOI] [PubMed] [Google Scholar]
- 11.Harris R, Trease L, Wilkie K, Drew M. Rib stress injuries in the 2012–2016 (Rio) Olympiad: a cohort study of 151 Australian Rowing Team athletes for 88 773 athlete days. Br J Sports Med. 2020;54(16):991–6. [DOI] [PubMed] [Google Scholar]
- 12.Barry DW, Kohrt WM. Acute effects of 2 hours of moderate-intensity cycling on serum parathyroid hormone and calcium. Calcif Tissue Int. 2007;80(6):359–65. [DOI] [PubMed] [Google Scholar]
- 13.Guillemant J, Accarie C, Peres G, Guillemant S. Acute effects of an oral calcium load on markers of bone metabolism during endurance cycling exercise in male athletes. Calcif Tissue Int. 2004;74(5):407–14. [DOI] [PubMed] [Google Scholar]
- 14.Barry DW, Hansen KC, van Pelt RE, Witten M, Wolfe P, Kohrt WM. Acute calcium ingestion attenuates exercise-induced disruption of calcium homeostasis. Med Sci Sports Exerc. 2011;43(4):617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haakonssen EC Ross ML Knight EJ, et al. The effects of a calcium-rich pre-exercise meal on biomarkers of calcium homeostasis in competitive female cyclists: a randomised crossover trial. PLoS One. 2015;10(5):e0123302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shea KL, Barry DW, Sherk VD, Hansen KC, Wolfe P, Kohrt WM. Calcium supplementation and parathyroid hormone response to vigorous walking in postmenopausal women. Med Sci Sports Exerc. 2014;46(10):2007–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wherry SJ Swanson CM Wolfe P, et al. Bone biomarker response to walking under different thermal conditions in older adults. Med Sci Sports Exerc. 2019;51(8):1599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wherry SJ, Blatchford PJ, Swanson CM, Wellington T, Boxer RS, Kohrt WM. Maintaining serum ionized calcium during brisk walking attenuates the increase in bone resorption in older adults. Bone. 2021;153:116108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohrt WM Wolfe P Sherk VD, et al. Dermal calcium loss is not the primary determinant of parathyroid hormone secretion during exercise. Med Sci Sports Exerc. 2019;51(10):2117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klesges RC Ward KD Shelton ML, et al. Changes in bone mineral content in male athletes. Mechanisms of action and intervention effects. JAMA. 1996;276(3):226–30. [PubMed] [Google Scholar]
- 21.Kohrt WM Wherry SJ Wolfe P, et al. Maintenance of serum ionized calcium during exercise attenuates parathyroid hormone and bone resorption responses. J Bone Miner Res. 2018;33(7):1326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Townsend R Elliott-Sale KJ Pinto AJ, et al. Parathyroid hormone secretion is controlled by both ionized calcium and phosphate during exercise and recovery in men. J Clin Endocrinol Metab. 2016;101(8):3231–9. [DOI] [PubMed] [Google Scholar]
- 23.Scott JP, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD. Effect of recovery duration between two bouts of running on bone metabolism. Med Sci Sports Exerc. 2013;45(3):429–38. [DOI] [PubMed] [Google Scholar]
- 24.Bouassida A Zalleg D Zaouali Ajina M, et al. Parathyroid hormone concentrations during and after two periods of high intensity exercise with and without an intervening recovery period. Eur J Appl Physiol. 2003;88(4–5):339–44. [DOI] [PubMed] [Google Scholar]
- 25.Fensham NC McKay AKA Tee N, et al. Sequential submaximal training in elite male rowers does not result in amplified increases in interleukin-6 or hepcidin. Int J Sport Nutr Exerc Metab. 2022;32(3):177–85. [DOI] [PubMed] [Google Scholar]
- 26.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2):92–8. [PubMed] [Google Scholar]
- 27.Nana A Slater GJ Hopkins WG, et al. Importance of standardized DXA protocol for assessing physique changes in athletes. Int J Sport Nutr Exerc Metab. 2016;26(3):259–67. [DOI] [PubMed] [Google Scholar]
- 28.Nana A, Slater GJ, Hopkins WG, Burke LM. Techniques for undertaking dual-energy x-ray absorptiometry whole-body scans to estimate body composition in tall and/or broad subjects. Int J Sport Nutr Exerc Metab. 2012;22(5):313–22. [DOI] [PubMed] [Google Scholar]
- 29.Jeacocke NA, Burke LM. Methods to standardize dietary intake before performance testing. Int J Sport Nutr Exerc Metab. 2010;20(2):87–103. [DOI] [PubMed] [Google Scholar]
- 30.Van Beaumont W, Greenleaf JE, Juhos L. Disproportional changes in hematocrit, plasma volume, and proteins during exercise and bed rest. J Appl Physiol. 1972;33(1):55–61. [DOI] [PubMed] [Google Scholar]
- 31.McKay AKA Stellingwerff T Smith ES, et al. Defining training and performance caliber: a participant classification framework. Int J Sports Physiol Perform. 2022;17(2):317–31. [DOI] [PubMed] [Google Scholar]
- 32.Ogan D, Pritchett K. Vitamin D and the athlete: risks, recommendations, and benefits. Nutrients. 2013;5(6):1856–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adlercreutz H Härkönen M Kuoppasalmi K, et al. Effect of training on plasma anabolic and catabolic steroid hormones and their response during physical exercise. Int J Sports Med. 1986;7(1 Suppl):27–8. [DOI] [PubMed] [Google Scholar]
- 34.Sherk VD, Wherry SJ, Barry DW, Shea KL, Wolfe P, Kohrt WM. Calcium supplementation attenuates disruptions in calcium homeostasis during exercise. Med Sci Sports Exerc. 2017;49(7):1437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trease L, Wilkie K, Lovell G, Drew M, Hooper I. Epidemiology of injury and illness in 153 Australian international-level rowers over eight international seasons. Br J Sports Med. 2020;54(21):1288–93. [DOI] [PubMed] [Google Scholar]
- 36.Dimitriou L Weiler R Lloyd-Smith R, et al. Bone mineral density, rib pain and other features of the female athlete triad in elite lightweight rowers. BMJ Open. 2014;4(2):e004369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silla KEJ, Brigham SK, Goldstein M, Misra M, Singhal V. Clinical, biochemical, and hematological characteristics of community-dwelling adolescent and young adult males with anorexia nervosa. Int J Eat Disord. 2021;54(12):2213–7. [DOI] [PubMed] [Google Scholar]
- 38.Vinther A Kanstrup IL Christiansen E, et al. Exercise-induced rib stress fractures: influence of reduced bone mineral density. Scand J Med Sci Sports. 2005;15(2):95–9. [DOI] [PubMed] [Google Scholar]
- 39.Mathis SL, Farley RS, Fuller DK, Jetton AE, Caputo JL. The relationship between cortisol and bone mineral density in competitive male cyclists. J Sports Med (Hindawi Publ Corp). 2013;2013:896821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dolan E, Varley I, Ackerman KE, Pereira RMR, Elliott-Sale KJ, Sale C. The bone metabolic response to exercise and nutrition. Exerc Sport Sci Rev. 2020;48(2):49–58. [DOI] [PubMed] [Google Scholar]
- 41.Sherk VD, Barry DW, Villalon KL, Hansen KC, Wolfe P, Kohrt WM. Bone loss over 1 year of training and competition in female cyclists. Clin J Sport Med. 2014;24(4):331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haakonssen EC Ross ML Cato LE, et al. Dairy-based preexercise meal does not affect gut comfort or time-trial performance in female cyclists. Int J Sport Nutr Exerc Metab. 2014;24(5):553–8. [DOI] [PubMed] [Google Scholar]
- 43.Silva BC, Bilezikian JP. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharmacol. 2015;22:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonjour JP, Kohrt W, Levasseur R, Warren M, Whiting S, Kraenzlin M. Biochemical markers for assessment of calcium economy and bone metabolism: application in clinical trials from pharmaceutical agents to nutritional products. Nutr Res Rev. 2014;27(2):252–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ivaska KK, Hentunen TA, Vaaraniemi J, Ylipahkala H, Pettersson K, Vaananen HK. Release of intact and fragmented osteocalcin molecules from bone matrix during bone resorption in vitro. J Biol Chem. 2004;279(18):18361–9. [DOI] [PubMed] [Google Scholar]
- 46.Wang JS, Mazur CM, Wein MN. Sclerostin and osteocalcin: candidate bone-produced hormones. Front Endocrinol (Lausanne). 2021;12:584147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parker L, Shaw CS, Byrnes E, Stepto NK, Levinger I. Acute continuous moderate-intensity exercise, but not low-volume high-intensity interval exercise, attenuates postprandial suppression of circulating osteocalcin in young overweight and obese adults. Osteoporos Int. 2019;30(2):403–10. [DOI] [PubMed] [Google Scholar]
- 48.Hiam D Landen S Jacques M, et al. Osteocalcin and its forms respond similarly to exercise in males and females. Bone. 2021;144:115818. [DOI] [PubMed] [Google Scholar]
- 49.Fensham NC, Heikura IA, McKay AKA, Tee N, Ackerman KE, Burke LM. Short-term carbohydrate restriction impairs bone formation at rest and during prolonged exercise to a greater degree than low energy availability. J Bone Miner Res. 2022;37(10):1915–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heikura IA Burke LM Hawley JA, et al. A short-term ketogenic diet impairs markers of bone health in response to exercise. Front Endocrinol (Lausanne). 2020;10:880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rantalainen T, Heinonen A, Linnamo V, Komi PV, Takala TE, Kainulainen H. Short-term bone biochemical response to a single bout of high-impact exercise. J Sports Sci Med. 2009;8(4):553–9. [PMC free article] [PubMed] [Google Scholar]


